Abstract
Background: Most pts with MM are older (median age at diagnosis: 69 y), have comorbidities (Engelhardt, et al. Haematologica. 2017;102:910), and often relapse. Thus, there is a high unmet need for novel, tolerable agents to treat RRMM. Melphalan flufenamide (melflufen)-a first-in-class alkylating peptide-drug conjugate targeting aminopeptidases-and dex received accelerated approval for use in RRMM in the United States based on the phase 2 HORIZON study results (Richardson, et al. J Clin Oncol. 2021;39:757). Melflufen + dex was superior to pom + dex in pts with RRMM based on the primary endpoint of progression-free survival (hazard ratio [HR], 0.79 [95% CI, 0.64-0.98]; P=.0311), but showed no overall survival benefit (HR, 1.10 [95% CI, 0.85-1.44]) in the phase 3 randomized OCEAN study (NCT03151811; Oncopeptides. Press release. Jul 8, 2021). This abstract reports the first high-level safety and tolerability data from OCEAN.
Methods: Pts with RRMM (2-4 prior lines of therapy [LoTs], including lenalidomide [len] and a proteasome inhibitor) refractory to len (within 18 mo of randomization) and last LoT were randomized 1:1 to 28-d cycles of melflufen 40 mg intravenously (d1) + dex 40 mg (20 mg for pts ≥75 y) orally (PO) on d1, 8, 15, and 22 or pom 4 mg/d PO (d1 to 21) + dex (dosage as in melflufen arm). Safety and tolerability were assessed by incidence and severity of adverse events (AEs) and presented by MedDRA Preferred Terms (PTs). Hematologic AEs, infections, and hemorrhages were AEs of special interest, identified by either Standardised MedDRA Queries or grouping of similar PTs, unless indicated otherwise. Dose modifications of melflufen and pom were permitted to manage AEs.
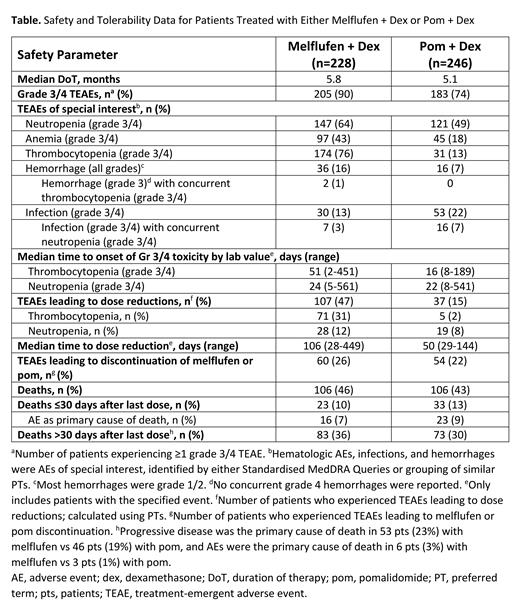
Results: As of 3 Feb 2021, 228/246 (93%) and 246/249 (99%) pts in the melflufen and pom arms, respectively, received ≥1 dose of study drug and were included in the safety analysis (Table). Median duration of therapy was 5.8 mo with melflufen and 5.1 mo with pom. Treatment-emergent AEs (TEAEs) grade (Gr) 3/4 were experienced by 90% and 74% of pts with melflufen and pom, respectively. The most common Gr 3/4 hematologic TEAEs were neutropenia (melflufen: 64% vs pom: 49%), anemia (43% vs 18%), and thrombocytopenia (76% vs 13%). With melflufen and pom, hemorrhages (mostly Gr 1/2) occurred in 16% vs 7% of pts; Gr 3 hemorrhages with concurrent Gr 3/4 thrombocytopenia occurred in 2 pts (1%) and 0 pts (no concurrent Gr 4 hemorrhages reported); Gr 3/4 infections occurred in 13% vs 22% of pts (concurrently with Gr 3/4 neutropenia in 3% vs 7%), respectively. TEAEs led to dose reductions in 47% with melflufen vs 15% with pom (most commonly, thrombocytopenia [31% vs 2%] and neutropenia [12% vs 8%]; as PTs) and permanent discontinuation of melflufen/pom in 26% vs 22%, respectively. Median time (range) to dose reduction was 106 d (28-449) with melflufen vs 50 d (29-144) with pom. Median time (range) to onset of Gr 3/4 thrombocytopenia and Gr 3/4 neutropenia by lab values was 51 d (2-451) and 24 d (5-561) with melflufen vs 16 d (8-189) and 22 d (8-541) with pom, respectively. TEAEs resulting in dose modifications (reduction, hold, or discontinuation) occurred less frequently in older vs younger pts with melflufen (<65 y, 70/89 [79%]; ≥75 y, 24/37 [65%]), but more frequently in older pts with pom (<65 y, 45/82 [55%]; ≥75 y, 25/39 [64%]). The proportion of dose modifications was higher with melflufen than pom in pts aged <65 y (79% vs 55%) but was similar in both arms in pts aged ≥75 y (65% vs 64%). TEAEs led to discontinuations at similar rates in ages <65 and ≥75 y, respectively, with melflufen (26% vs 22%) but not with pom (20% vs 36%). With melflufen and pom, respectively, deaths occurred in 106 pts (46%) vs 106 pts (43%) overall, deaths ≤30 d after last dose occurred in 23 pts (10%) vs 33 pts (13%)-AE was the primary cause of death in 16 pts (7%) vs 23 pts (9%)-and deaths >30 d after last dose occurred in 83 pts (36%) vs 73 pts (30%)-primarily due to progressive disease in both arms.
Conclusion: In the OCEAN study, safety and tolerability of melflufen + dex was consistent with prior reports. Hematologic AEs were common but generally manageable with dose modifications. Despite a higher cytopenia frequency with melflufen, rates of concurrent Gr 3/4 bleedings and infections were low and Gr 3/4 infections were more common with pom. Discontinuation rates due to AEs were similar in both arms. These analyses show that melflufen + dex is generally safe and manageable in pts with RRMM, including in pts aged ≥75 y.
Richardson: Secura Bio: Consultancy; Regeneron: Consultancy; Karyopharm: Consultancy, Research Funding; Protocol Intelligence: Consultancy; Sanofi: Consultancy; Celgene/BMS: Consultancy, Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy; GlaxoSmithKline: Consultancy; Takeda: Consultancy, Research Funding; Janssen: Consultancy; Oncopeptides: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Schjesvold: Sanofi: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Oncopeptides: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; SkyliteDX: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Bayer: Consultancy; Nordics Nanovector: Current holder of individual stocks in a privately-held company; GSK: Research Funding; Adaptive Biotechnologies: Consultancy; Schain: Honoraria. Abdulhaq: BMS, Alexion, Oncopeptides, Morphosys, Pfizer, Norvartis: Honoraria; Oncopeptides, Alexion, Amgen: Speakers Bureau; Morphosys, BMS, Amgen: Membership on an entity's Board of Directors or advisory committees. Coriu: Amgen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses. Sonneveld: Janssen: Consultancy, Honoraria, Research Funding; SkylineDx: Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Kullenburg: Oncopeptides AB: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses ; Swedish Orphan Biovitrum AB: Ended employment in the past 24 months. Harmenberg: Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses . Thuresson: Oncopeptides AB: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Byrne: BMS: Current holder of individual stocks in a privately-held company; Oncopeptides AB: Current Employment, Current holder of stock options in a privately-held company. Mateos: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Yes, this abstract includes a subgroup analysis of a phase 3 investigational study of melflufen in patients with RRMM refractory to lenalidomide

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal